Software as a Medical Device (SaMD)
We specialize in the development and verification of standalone software solutions that function as medical devices. Our team ensures SaMD meets rigorous FDA, IEC 62304, and ISO 13485 requirements for safety, security, and reliability.
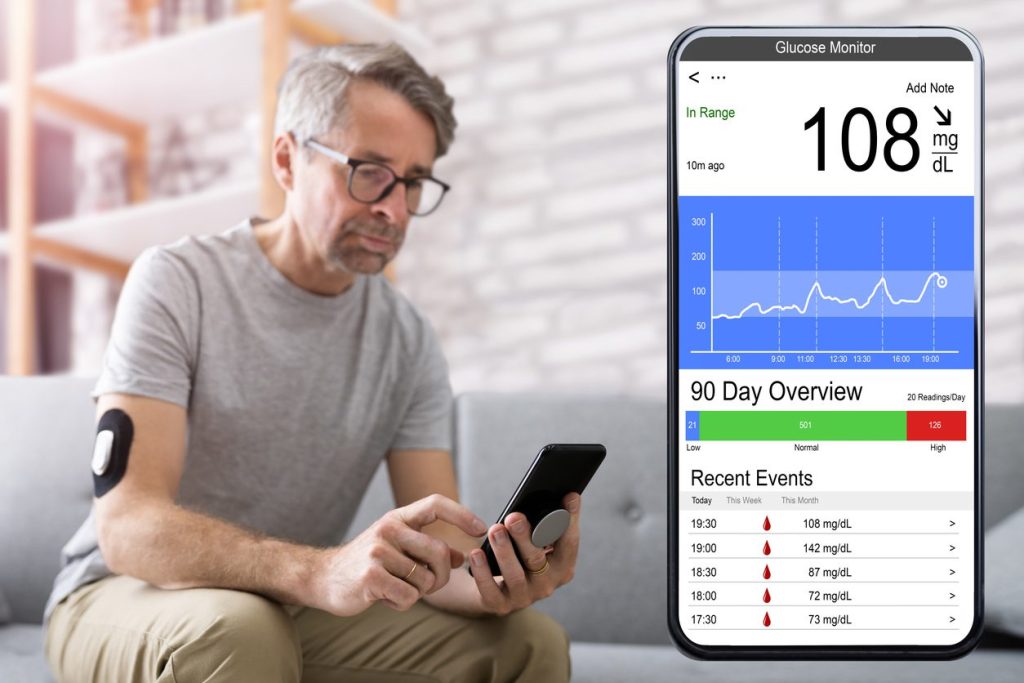
Digital Medicine & Digital Therapeutics (DTx)
We support the design and validation of software-driven medical interventions, including:
- Remote patient monitoring solutions that collect, analyze, and transmit physiological data
- Prescription digital therapeutics (PDTs) delivering evidence-based interventions for disease treatment and management
- AI-powered diagnostic tools that enhance clinical decision-making
Mobile & Cloud-Based Health Solutions
Our development capabilities include cloud-integrated platforms and mobile health applications. We specialize in secure data exchange, interoperability, and real-time patient engagement. While ensuring compliance with HIPAA, GDPR, and FDA, we develop for:
- Apple™ iOS using Swift and Objective-C in Xcode
- Google™ Android™ OS using Kotlin™ and Java™ in Android Studio™
- Cloud-based infrastructures, with experience in Microsoft Azure
Our team leverages industry-leading frameworks, testing tools, and regulatory expertise to deliver safe, effective, and compliant digital health solutions.


